
August, the vacation month. However, for winemaking enthusiasts, it is the month for preparing and beginning the harvest.
Starting with the characterization of the raw material, sampling the plots helps us to organize ourselves and know what to expect from the grapes this year.
The work done throughout the year begins to bear fruit. However, the treatments applied during this hot and rainy year will influence the future must. The humidity was implanted months ago in the vineyard, and has led to the proliferation of mildew, with its subsequent damage to the fruit. But it is not just grape losses that are a problem. Phytosanitary treatments based on copper sulfate compounds remain in the vineyard and copper, for example, is absorbed by the plant.
Heavy metals such as copper are trace elements, having an important role in the redox balance of the must or as elements necessary for the transfer of electrons in mitochondria. However, if these elements are at higher levels, they cause collapse and oxidation in musts and wines, causing irreversible damage.
So far we have analyzed copper in wine for regulatory reasons, before bottling. After more than 10 years of research, we can say that copper begins its negative action in musts, since in alcoholic fermentation it is fixed on the yeast walls and practically not detected in finished wine. That is why it is difficult for us to find a bottled wine with concentrations higher than permitted.
In must, oxidation occurs enzymatically, specifically by polyphenol oxidases. We have mentioned more than once the damage that polyphenols cause in whites, forming quinones by enzymatic action that then polymerize, being responsible for browning in white wines. This reaction is catalyzed by copper, so the more metal we have, the more it will help the oxidation of the wine.
In addition, Copper is a well-known antifungal and antibacterial, which can affect the development of certain strains of Saccharomyces Cerevisiae increasing the latency phase and slowing down the fermentation speed. Copper can even affect lactic acid bacteria, reducing the breakdown of malic acid.
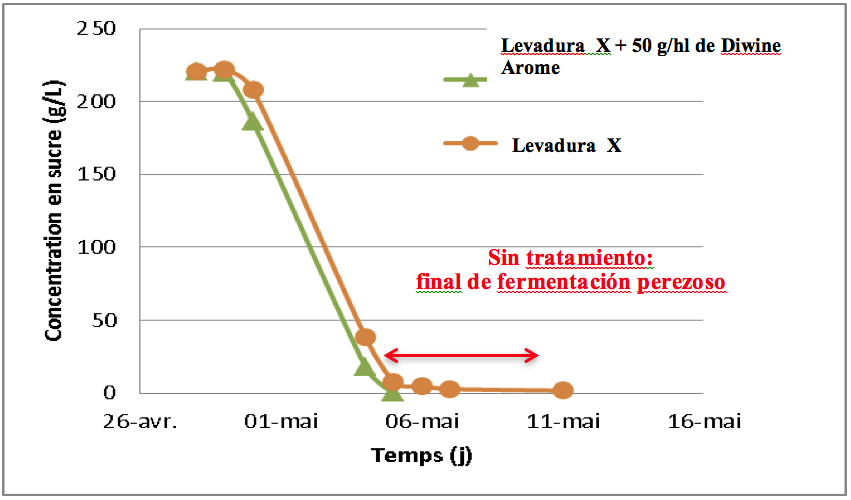
The most important use of copper in the winery is for its oxidative effect on wines presenting a reduction. The appearance of these types of aromas in wines can have different origins in order to prevent them:
- Nutritional problems during alcoholic fermentation. For good, safe fermentation with no risk of aromatic deviations, the must must have a minimum of 150 mg/L YAN. Depending on the alcoholic degree and the amount of NFA that the grape provides, it is necessary to correct so that the yeast works correctly and to adapt its nutritional needs with different types of nutrients. This means we can ensure that the yeast is not going to synthesize hydrogen sulfide compounds and that we have a quality aromatic range.
- Phenolic reduction. Normally, in years with freezing, the grape does not reach the desired phenolic maturity and the tannins are very reactive. They demand a lot of oxygen for them to polymerize and change to a higher structure. If we do not give them enough oxygen, the tannin tends to decrease and leave dryness in the mouth. Micro oxygenation is the key element in this type of wine so that its full aromatic potential is expressed.
- Reductions by lees: Lees have an important reducing effect, so when working with them it is necessary to lift them frequently or keep them suspended with the help of a stirrer. As they settle to the bottom of the tank, the pressure exerted helps them produce sulfur compounds that mask the fruit of the wine.
Other heavy metals that we cannot ignore are iron and aluminum. In this case, one of the origins of these metals is certain types of bentonites. They contain Fe and Al cations in interlaminar positions to later give rise to a cationic exchange together with the proteins in the wines. In this exchange, the metals are transferred to the wine and are capable of accelerating browning reactions. Iron is able to oxidize ethanol and tartaric acid, creating molecules that combine with the free sulfur in the wine, reducing its efficacy and leaving the wine unprotected. The decrease in free sulfur can accelerate the evolution of bottled wines, even causing loss of their aromatic quality.
So we cannot ignore the presence of heavy metals and let them act in the must; We continue to strive so that the wine reaches our consumer as it should.
Related news
THE FRESHNESS OF THE WINE, from the bunch to the glass
The fashion for fresh wines has ceased to be fashion to become a trend; more and more consumers are looking for elegance, length and freshness above all.
More than winemaking machines
The winemaker is the artist who observes the vineyard, interprets it and imagines the wine that could be produced from this plot.
Was it the wood or the process?
Today, we are thinking about one of the most important stages of production: the aging of the wine.


